Dec 2024 Edition

Impact Factor 2023
All About Combination Therapies From ISN Journals
This collection of articles from Kidney International® and Kidney International Reports® focuses on innovative treatment protocols using combination therapies.

KIDNEY INTERNATIONAL ARTICLES |
Results of a Randomized Double-blind Placebo-controlled Phase 2 Study Propose Iptacopan as an Alternative Complement Pathway Inhibitor for IgA Nephropathy

Targeting the alternative complement pathway is emerging as a promising therapeutic approach for immunoglobulin A nephropathy (IgAN) due to its role in disease progression. In a recently published Phase 2 study, iptacopan — an oral inhibitor targeting factor B of the alternative complement pathway — was evaluated in patients with biopsy-confirmed IgAN.
Patients were randomly assigned to receive one of four doses of iptacopan or a placebo. Results demonstrated a significant, dose-dependent reduction in proteinuria at three and six months, particularly in the higher-dose groups.
Iptacopan was well-tolerated with no serious adverse events and effectively reduced key complement biomarkers. These findings support the continued evaluation of iptacopan in an ongoing Phase 3 trial.
Clinical Trial Designs to Assess Treatment Effects on Glomerular Filtration Rate Decline
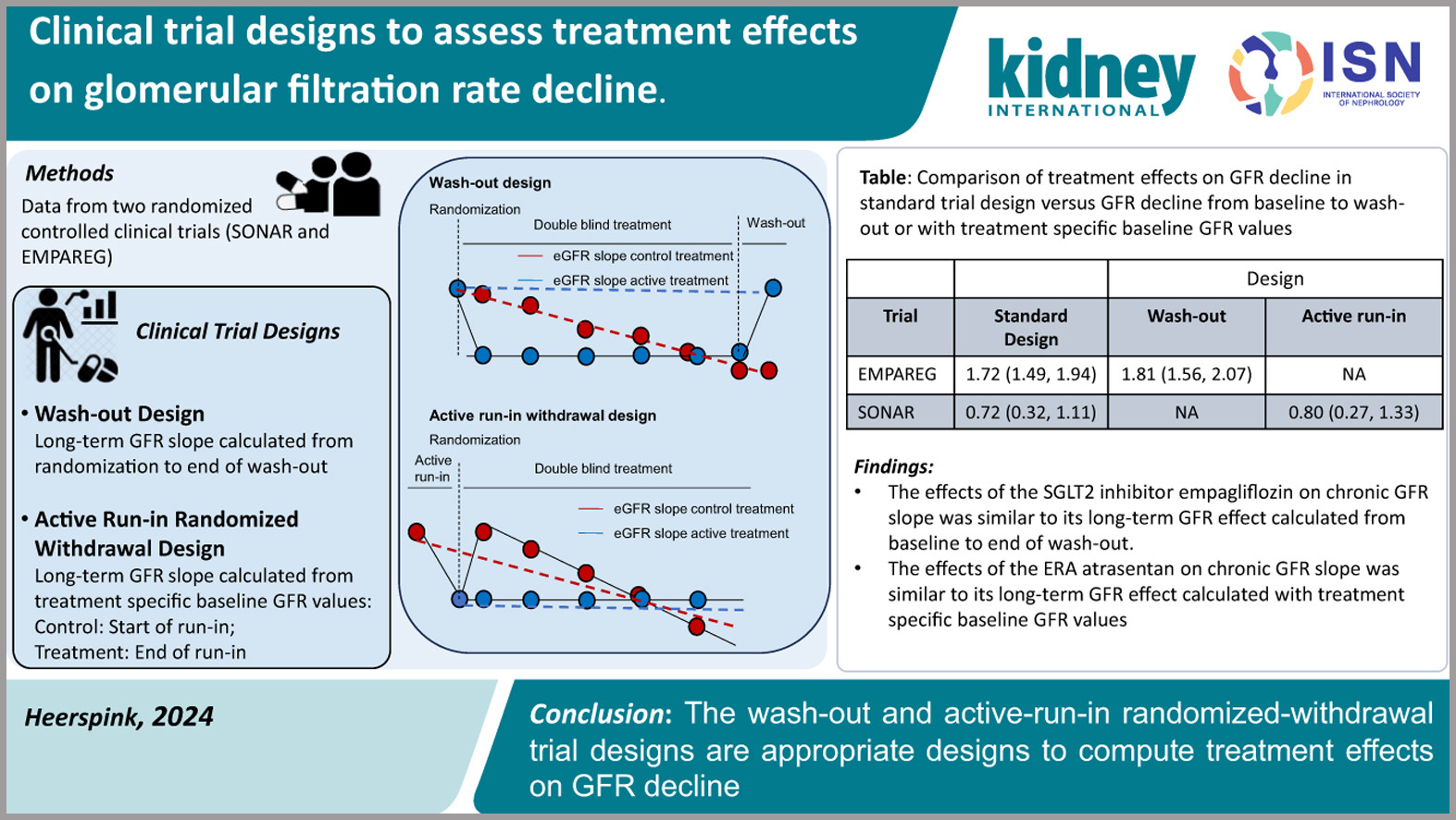
Traditional clinical trials aimed at delaying chronic kidney disease (CKD) progression have relied on endpoints such as a 57% decline in glomerular filtration rate (GFR) (equivalent to a doubling of serum creatinine) and kidney failure. However, these endpoints require large, lengthy trials to detect significant differences, presenting logistical challenges. As a result, GFR slope has gained traction as a surrogate endpoint.
Interventions like renin-angiotensin-aldosterone system blockers and sodium-glucose cotransporter-2 inhibitors, while effective at slowing CKD progression, exhibit acute effects on GFR that differ from their longer-term impacts. This divergence complicates the prediction of long-term outcomes within standard trial follow-up periods.
To address this, the authors evaluated two alternative trial designs using data from the EMPA-REG Outcome and SONAR trials. The first approach involved a washout design, where GFR was measured off-treatment at the trial’s conclusion. The second employed an active run-in randomized withdrawal design. Both methods were compared to the conventional design, which calculates chronic GFR slope from the first on-treatment visit to the end of treatment.
The findings demonstrate that both washout and active run-in randomized withdrawal designs provided greater statistical power than the standard approach. The results suggest that these alternative designs are effective and robust models for assessing treatment effects on GFR decline in CKD trials.
A Randomized Phase 2b Trial Examined the Effects of the Glucagon-like Peptide-1 and Glucagon Receptor Agonist Cotadutide on Kidney Outcomes in Patients With Diabetic Kidney Disease
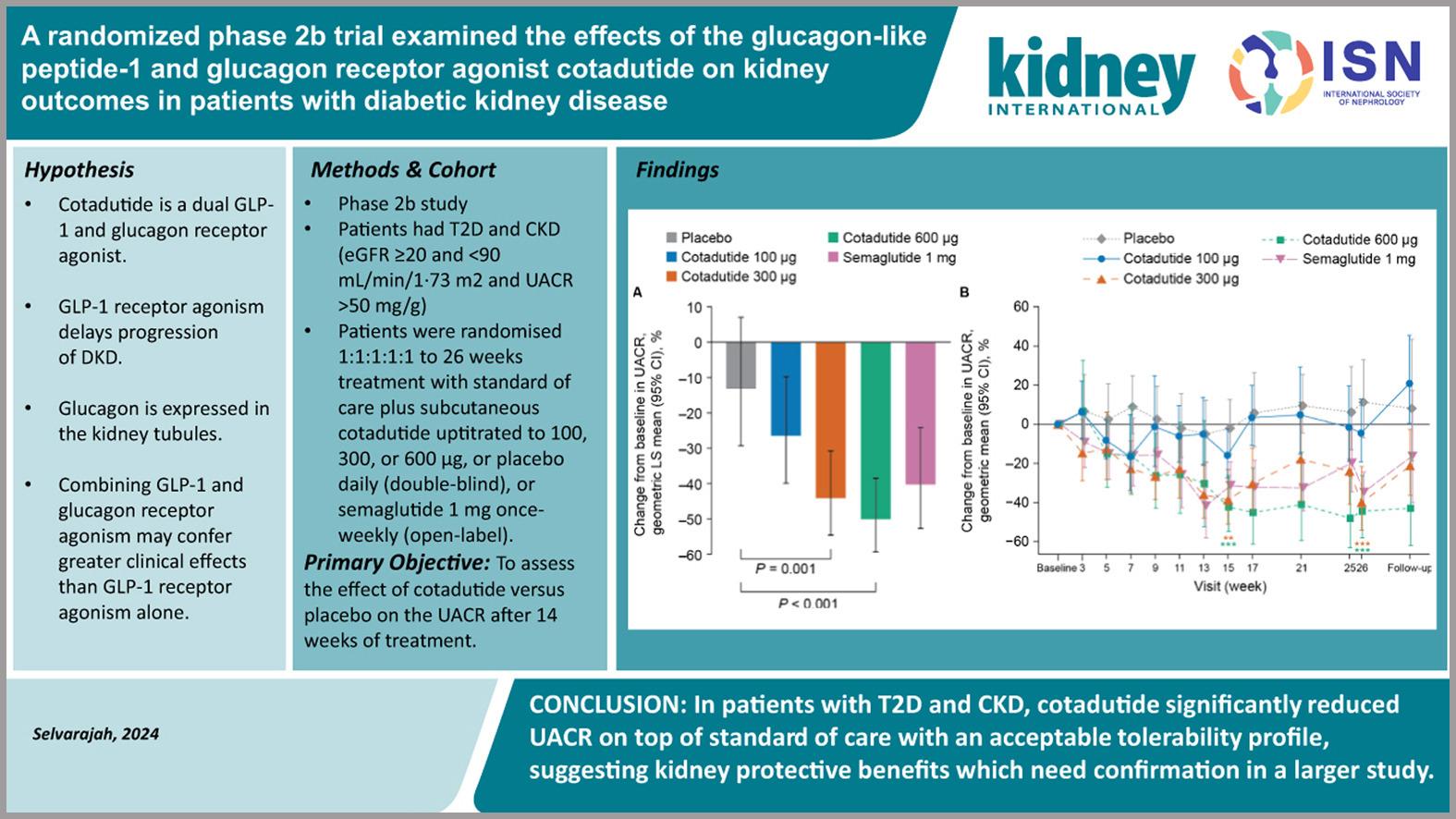
Diabetes mellitus is a leading contributor to the global burden of kidney disease, and recent advancements in diabetic kidney disease (DKD) management continue to generate significant interest. While GLP-1 receptor agonists have already demonstrated renal and metabolic benefits, cotadutide introduces an innovative approach by combining GLP-1 and glucagon receptor activation.
Preliminary data suggest cotadutide has beneficial effects comparable to GLP-1 receptor agonists, including reductions in urinary albumin-to-creatinine ratio, even in patients with an estimated glomerular filtration rate (eGFR) as low as 20 mL/min/1.73 m². The rationale for incorporating glucagon receptor agonism lies in its potential kidney-specific benefits, given the expression of glucagon receptors in renal tubules.
Ongoing research will determine whether this dual-agonist approach offers additional advantages, such as enhanced eGFR preservation, improved glycemic control, weight management, and broader metabolic benefits. Larger, long-term studies are eagerly awaited to confirm these findings and define cotadutide’s role in DKD management.
A Population-based Cohort Defined Risk of Hyperkalemia After Initiating SGLT-2 Inhibitors, GLP1 Receptor Agonists or DPP-4 Inhibitors to Patients With Chronic Kidney Disease and Type 2 Diabetes
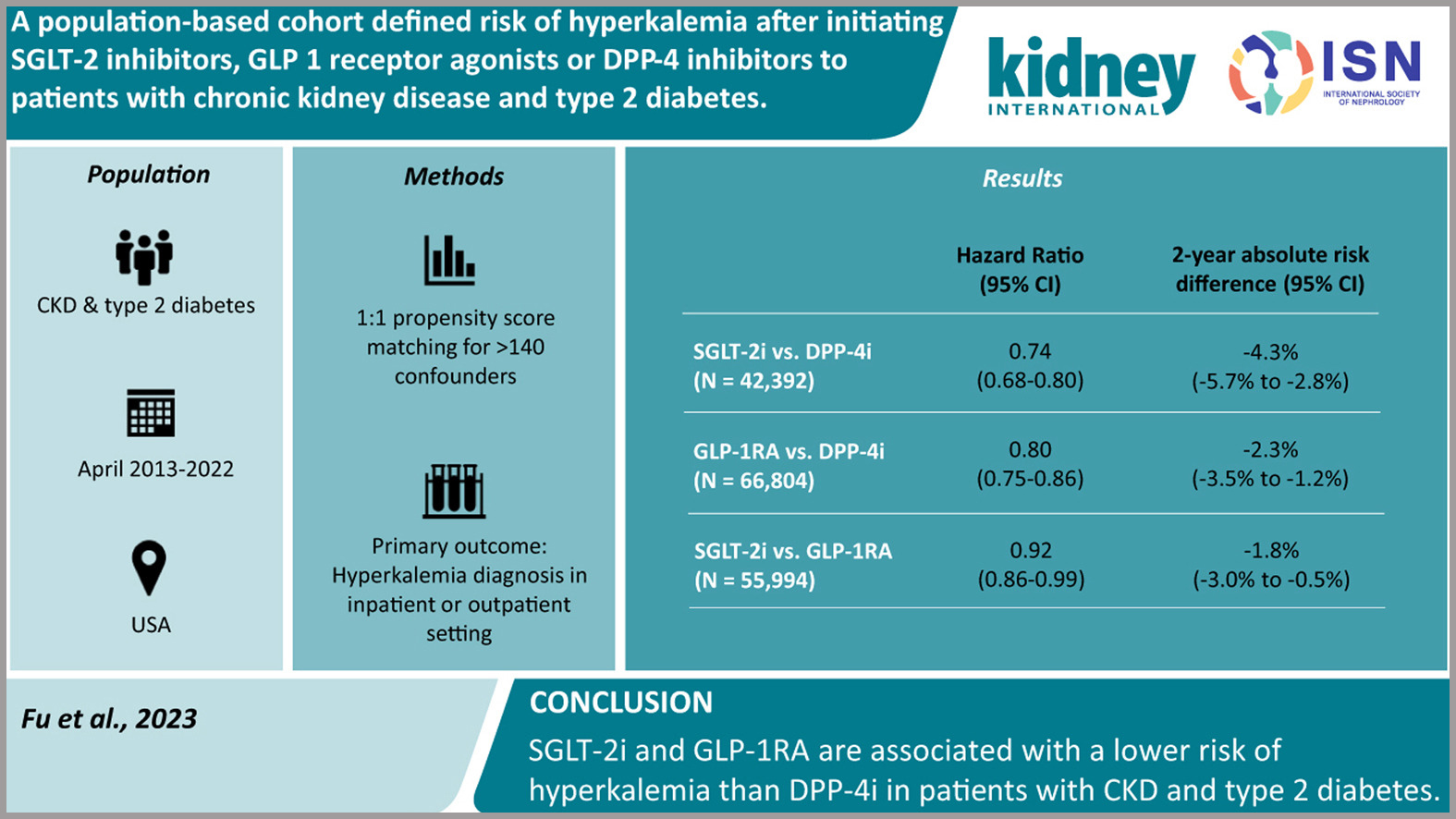
People with type 2 diabetes and kidney disease frequently experience hyperkalemia, which makes it challenging to continue taking nephroprotective medications such as angiotensin-converting enzyme inhibitors. In a population-based cohort trial, the scientists investigated the potential for novel glucose-lowering drugs to prevent hyperkalemia. The study included individuals with type 2 diabetes and CKD stages 3-4 who had just started taking SGLT-2i, GLP-1RA, or DPP-4i. Data was taken from Medicare and two sizable commercial insurance databases in the US between April 2013 and 2022.
SGLT-2i vs. DPP-4i (141671 patients), GLP-1RA vs. DPP-4i (159545 patients), and SGLT-2i vs. GLP-1RA (93033 patients) were included. Initiation of SGLT-2i was associated with a lower risk of hyperkalemia compared with DPP-4i (hazard ratio 0.74; 95% confidence interval 0.68-0.80) and contrasted with GLP-1RA (0.92; 0.86-0.99). Compared with DPP-4i, GLP-1RAs were also associated with a lower risk of hyperkalemia (0.80; 0.75-0.86).
According to this study, individuals with type 2 diabetes and kidney disease who used SGLT-2i and GLP-1RAs were less likely to experience hyperkalemia than those who began using DPP-4i. The use of these drugs in patients with renal disease and type 2 diabetes is supported by this study.
Anti-obesity Pharmacotherapy in Adults With Chronic Kidney Disease
This review underscores obesity as a key risk factor for chronic kidney disease (CKD) progression, influencing management and outcomes. While lifestyle interventions are foundational, their long-term success is often limited.
Advances in pharmacotherapy, particularly GLP-1 receptor agonists like liraglutide, semaglutide, and tirzepatide, are transforming obesity treatment, offering significant weight loss, metabolic improvements, and potential renoprotection. However, due to possible side effects, their use in CKD requires individualized dosing and monitoring.
Evidence in CKD populations remains limited, as most trials exclude patients with advanced renal impairment. The review highlights the need to tailor obesity management to CKD severity, combine pharmacotherapy with lifestyle changes, and explore the links between weight loss, metabolic health, and renal outcomes. Ongoing trials may refine treatment approaches.
KIDNEY INTERNATIONAL REPORTS ARTICLES |
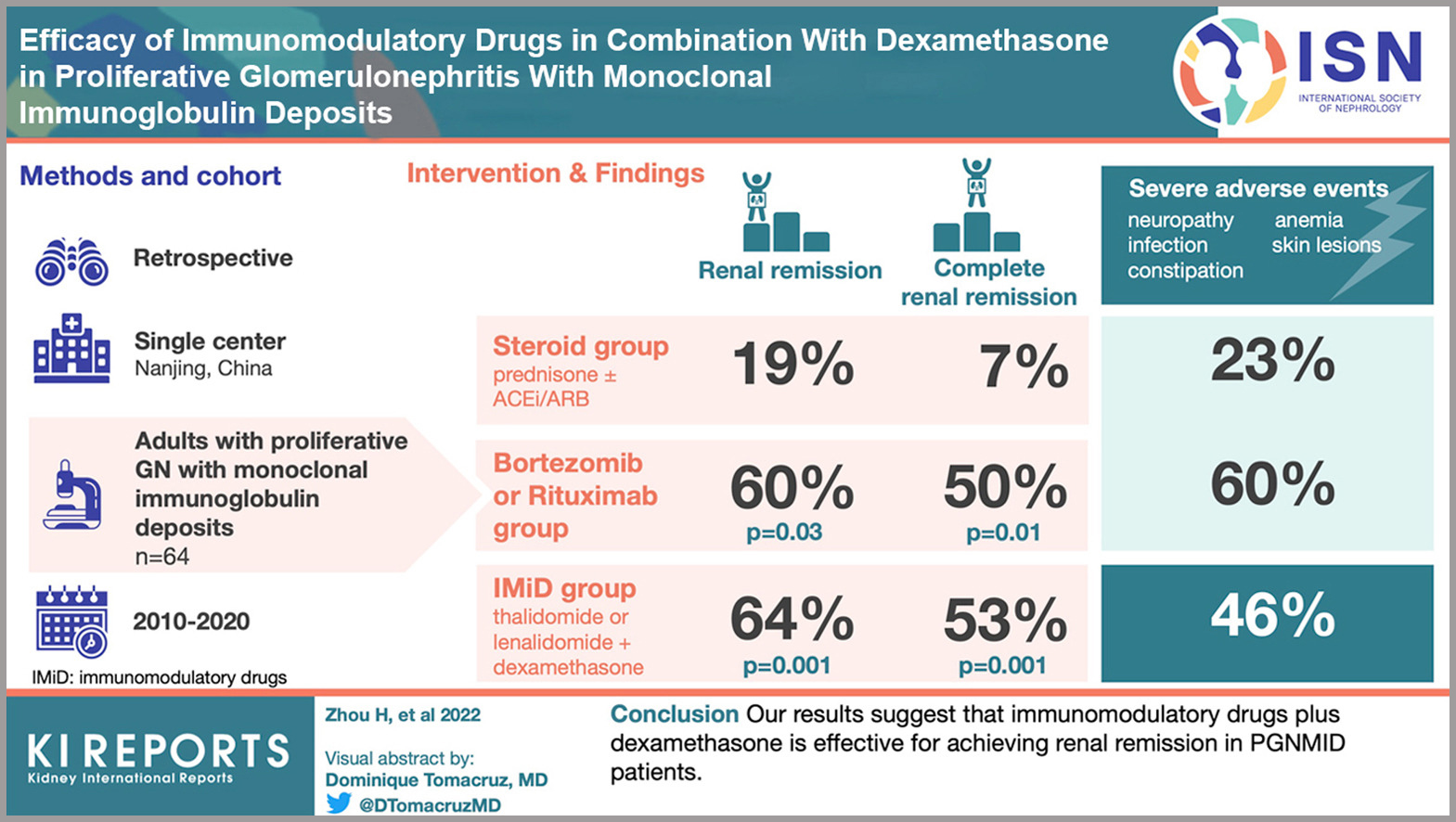
Immunomodulatory drugs (IMiDs) combined with dexamethasone offer a promising treatment for proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID), a rare and challenging kidney condition.
In a retrospective analysis of 64 PGNMID patients at the National Clinical Research Center of Kidney Disease in Nanjing, China, treatment with either IMiDs, steroids, or bortezomib/dexamethasone plus rituximab (BD/RTX) revealed that renal remission rates were notably higher in the IMiD and BD/RTX groups compared to the steroid group.
Poorer outcomes were linked to hypertension and elevated serum creatinine levels, whereas low C3 levels, IMiDs, and BD/RTX were associated with improved renal response. Although severe adverse events were more frequent in the IMiD and BD/RTX groups, IMiDs combined with dexamethasone remain an effective option for achieving renal remission in PGNMID patients.
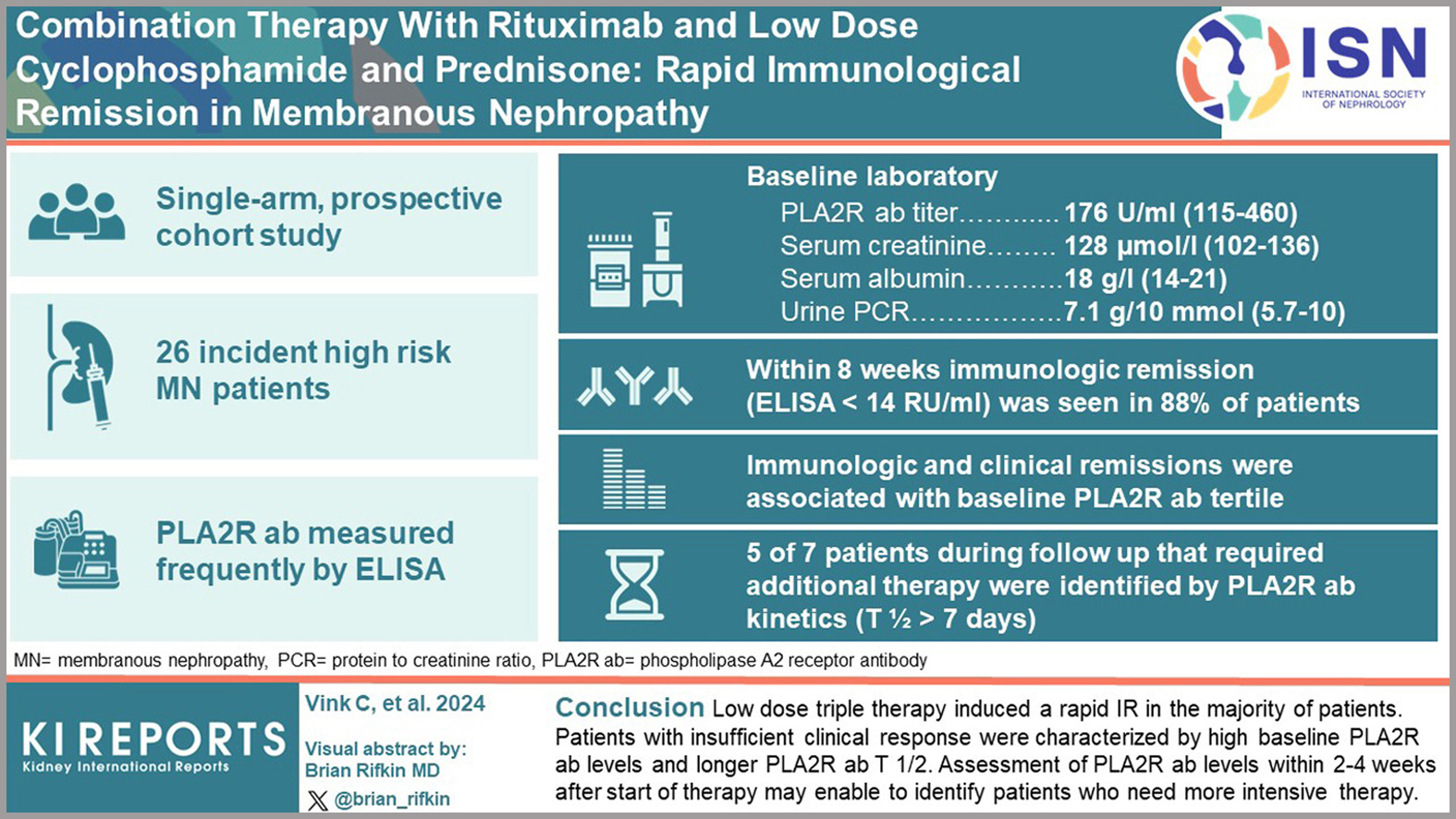
The current Kidney Disease: Improving Global Outcomes guidelines for treating membranous nephropathy emphasize a risk-stratified approach tailored to patient characteristics. Recommended options include rituximab or calcineurin inhibitors (CNIs) with or without glucocorticoids for moderate-risk patients, and rituximab, cyclophosphamide with glucocorticoids, or a combination of CNIs and rituximab for high-risk cases. For very high-risk patients, cyclophosphamide with glucocorticoids remains a key option. However, treatment failure and relapse remain significant challenges, particularly in high-risk patients.
A recent study explored a novel approach using a combination therapy of rituximab, low-dose cyclophosphamide, and prednisone in high-risk, PLA2R antibody-positive patients. The results demonstrate improved immunological and clinical responses in the majority of participants. Notable advantages include a reduced cumulative dose of cyclophosphamide, potentially lowering treatment-related toxicity, and lower rates of treatment failure and relapse compared to rituximab-based monotherapy.
These findings may pave the way for more individualized, patient-centered treatment strategies, offering renewed hope for better outcomes in this challenging patient population.
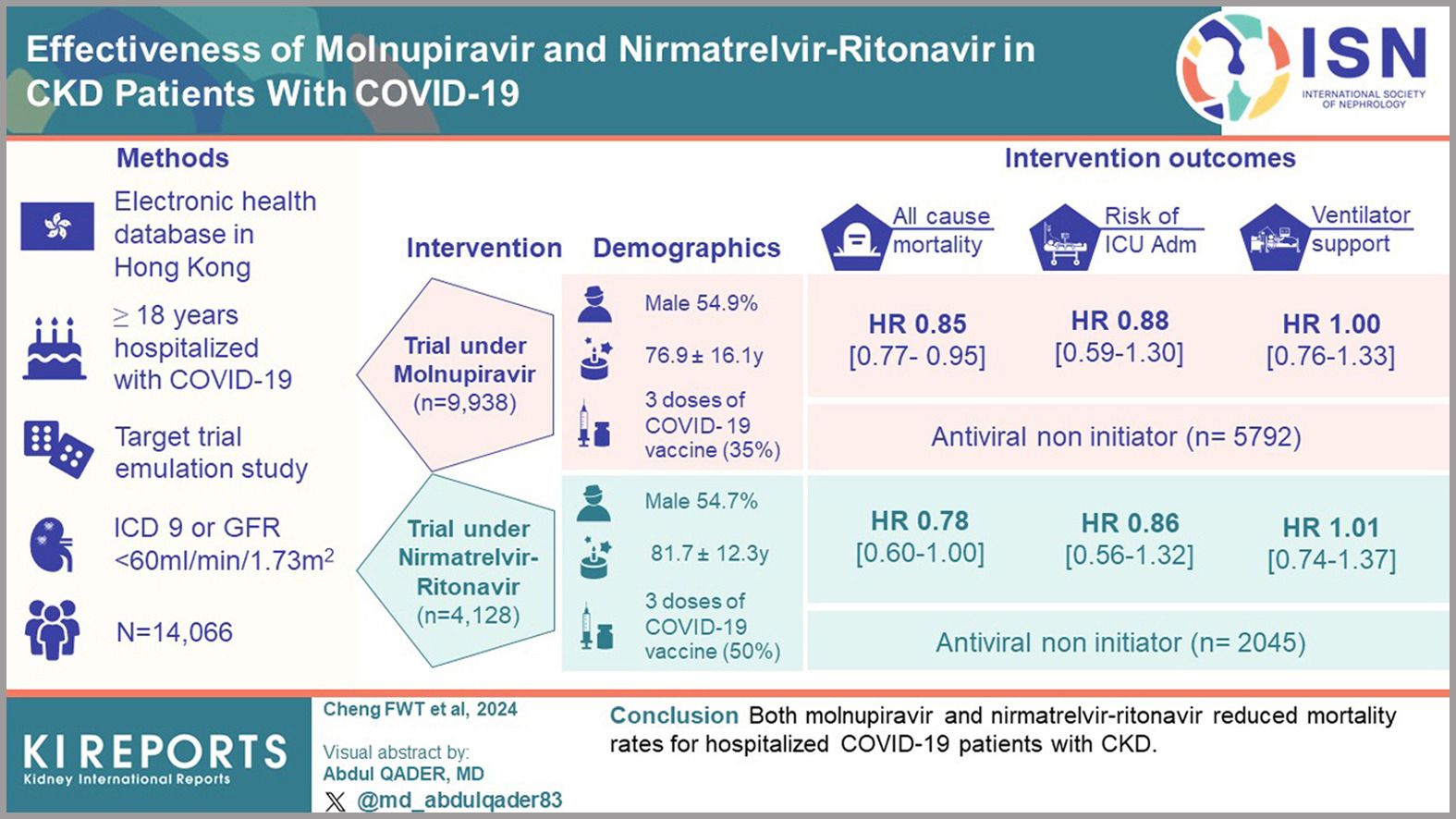
This target trial emulation study assessed the effectiveness of nirmatrelvir-ritonavir and molnupiravir in reducing mortality and severe COVID-19 outcomes among patients with chronic kidney disease (CKD). Data were sourced from Hong Kong’s electronic health databases, focusing on adult CKD patients.
The study demonstrated that antiviral treatments reduced the risk of all-cause mortality. Specifically, nirmatrelvir-ritonavir showed a hazard ratio (HR) of 0.78 (95% CI, 0.60-1.00), while molnupiravir had an HR of 0.85 (95% CI, 0.77-0.95). However, neither antiviral significantly decreased the risk of ventilatory support or admission to intensive care. Subgroup analysis revealed that patients with higher Charlson Comorbidity Index scores and male patients experienced more pronounced risk reductions. Moreover, among patients treated with nirmatrelvir-ritonavir, those who had received three or more vaccine doses showed a lower mortality risk.
The authors concluded that in hospitalized COVID-19 patients with CKD, both molnupiravir and nirmatrelvir-ritonavir were associated with reduced mortality. These findings highlight the potential benefits of antiviral therapies in this high-risk population.

Managing serum phosphorus is crucial for dialysis patients to reduce risks such as hyperparathyroidism and vascular calcification. While phosphate binders (PBs) are standard therapy, they often fall short in achieving adequate phosphorus control. Tenapanor, a selective inhibitor of intestinal sodium/hydrogen exchanger 3 transporters, offers a new mechanism to decrease passive phosphate absorption and improve phosphorus levels.
A recent study evaluated tenapanor in Japanese hemodialysis patients with hyperphosphatemia poorly controlled by PBs alone. The addition of tenapanor to PBs over 8 weeks resulted in a significant reduction in serum phosphorus levels compared to placebo (-2.00 mg/dL vs. -0.24 mg/dL; difference -1.76 mg/dL, P < 0.0001). While diarrhea was a common treatment-emergent adverse event (63.1% in the tenapanor group), it was mild to moderate in all cases, and no new safety concerns were identified.
These findings highlight tenapanor as a promising adjunctive therapy for hyperphosphatemia management, particularly for patients who do not achieve adequate control with PBs alone. Further research may solidify its role in clinical practice.

This open-label, prospective, single-center trial aimed to assess the effectiveness of hydroxychloroquine (HCQ) in conjunction with conventional immunotherapy for patients with primary membranous nephropathy.
From May 2020 to June 2024, 72 individuals with primary membranous nephropathy confirmed by biopsy were part of the study. At 3, 6, 9, and 12 months of follow-up, the authors examined the changes in proteinuria, serum albumin levels, estimated glomerular filtration rate, and relapse rate between 41 patients who received immunosuppressant and glucocorticoids and 31 patients who got HCQ with standard-of-care. Proteinuria was considerably lower in the HCQ group than in the standard-of-care group. At 6 months, there was less proteinuria (1.2 [0.4-2.2] vs. 2.2 [1.0-3.8] g/d, P ¼ 0.029), and the HCQ group saw a significantly lower relapse rate at 12 months than the standard-of-care group (3.7% vs. 23.3%, P ¼ 0.033).
The authors suggest that HCQ could be a useful adjunct treatment for primary membranous nephropathy.
